According to the claims, the device fractures when it is removed, resulting in catastrophic injuries and the need for surgery. Claimants contend that Paragard is defective and that the manufacturers failed to appropriately warn about the potential of device breakage and damage.
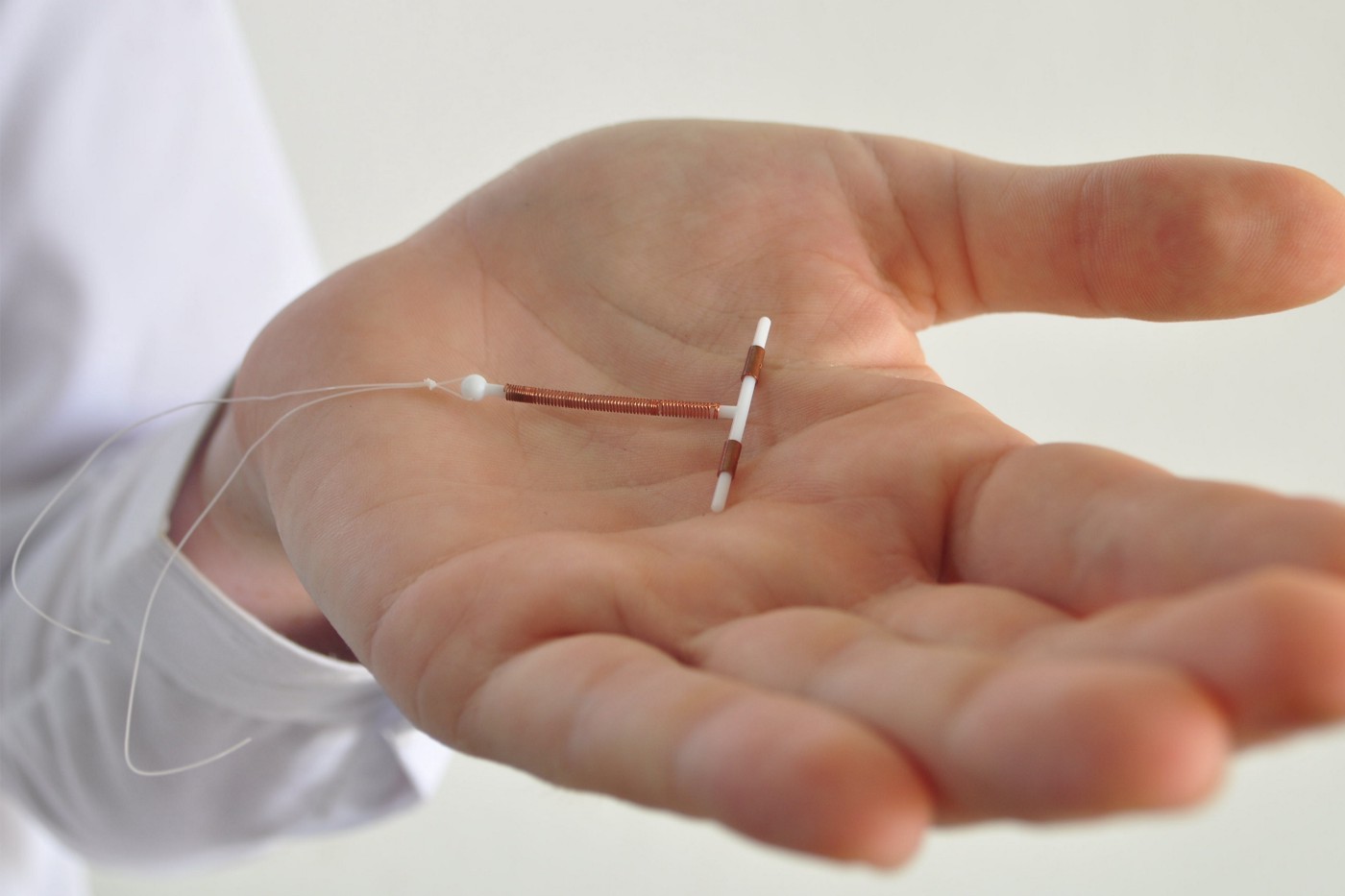
Pain, infertility, injuries from broken IUD pieces imbedding in organs, need for surgery
Teva Pharmaceuticals USA Inc., Teva Women’s Health Inc., Teva Women’s Health LLC, Teva Branded Pharmaceutical Products R&D Inc., The Cooper Companies Inc. and CooperSurgical Inc.
MDL 2974 in the Northern District of Georgia
- Perforation of the uterus or cervix
- Inflammations and allergic reactions to IUD pieces left in the body
- IUD migration
- IUD pieces missing or lodged in organs
- Infertility
- Need for surgery such as hysterectomy, laparoscopy or laparotomy
- Pain
- Infection
- Broken IUD pieces cannot be removed
Suits allege that the device broke when it was removed, resulting in injuries.
“Paragard removal is noninvasive and can be done by a healthcare professional during a typical office visit in just a few minutes,” according to Cooper Surgical’s website.
According to the instructions for removal given in the prescribing material, the device’s arms are designed to fold up when removed.
However, according to complaints filed by women, Paragard shattered during removal, leaving shards of the IUD in their bodies. The device had to be removed and issues treated in some women, which necessitated surgery.
Free Case Review
In December 2020, the Judicial Panel on Multidistrict Litigation in the Northern District of Georgia presided over by Judge Leigh Martin May, merged dozens of claims from across the country. Trials have yet to be scheduled as of January 28, 2021.


